Labforward unveils Labfolder go. Learn more in the press release
Making science flow.
Labforward transforms laboratories into intelligent workspaces with smart digital solutions.
- Labfolder: Intuitive Electronic Lab Notebook Software
- Labregister: Modern Lab Inventory Management Software
- Laboperator: Smart Laboratory Execution System
- Labtwin: AI- and Voice-Powered Digital Lab Assistant




-Photoroom.png?width=234&height=186&name=MPG_transparent_0_119_117%20(1)-Photoroom.png)







Labforward unveils Labfolder Go.
A fully integrated voice-powered mobile app to the Labfolder Electronic Lab Notebook (ELN), making it the world’s first ELN to provide out-of-the-box smart mobile assistance. Read more about it in our Press release.
ELN, IMS, LES, Digital Lab Assistant
Designed to uphold the highest standards of privacy, security, and data protection. We are certified under ISO 9001:2015 and ISO 27001:2017 and have successfully completed the SOC 2 Type 2 examination.
- Electronic lab notebook
- Inventory Management System
- Lab Execution System
- Digital Lab Assistant
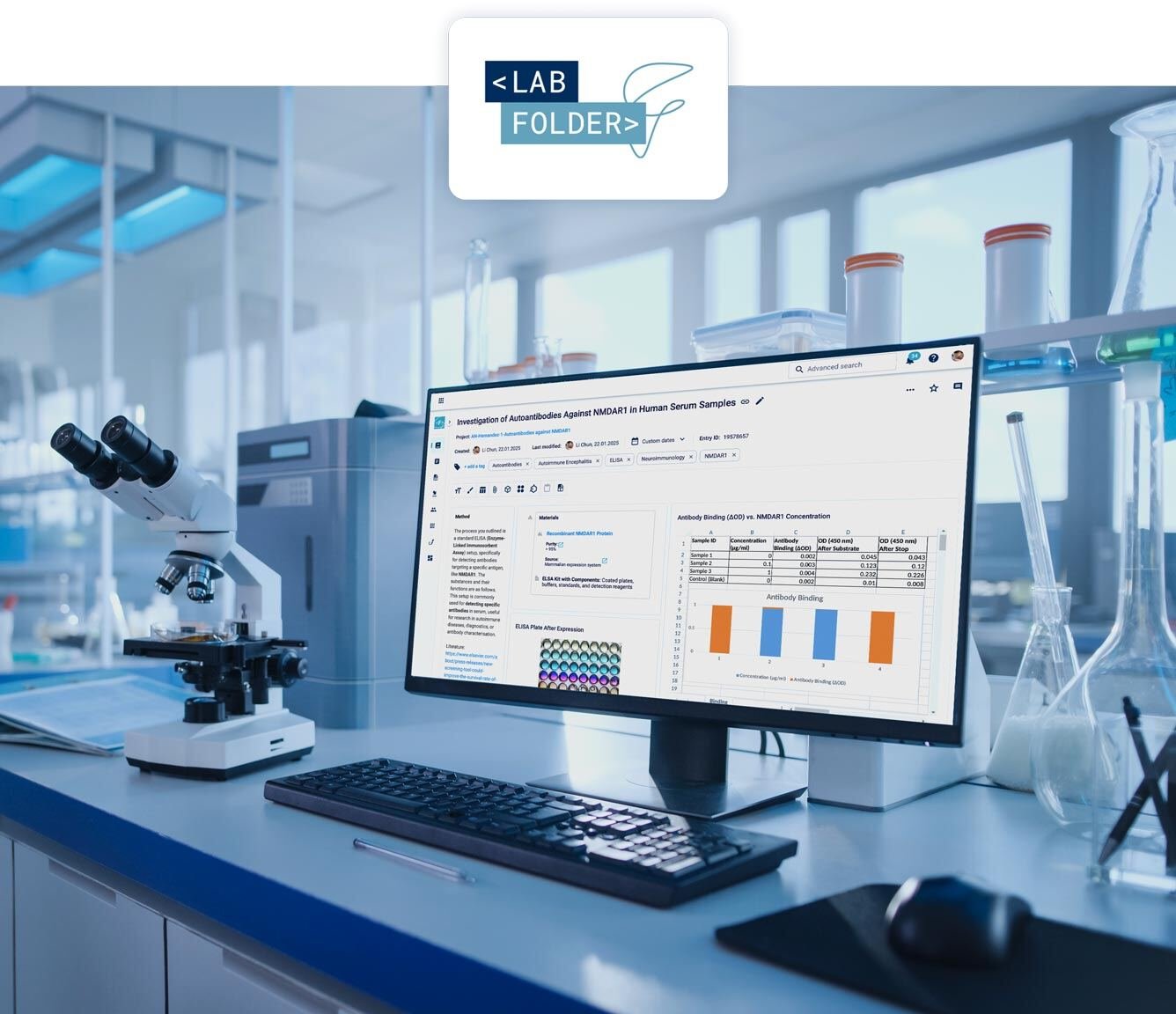
Your lab notes, always within reach.
-
No installation required
-
All your data and protocols in one place
-
Secure, compliant and scalable
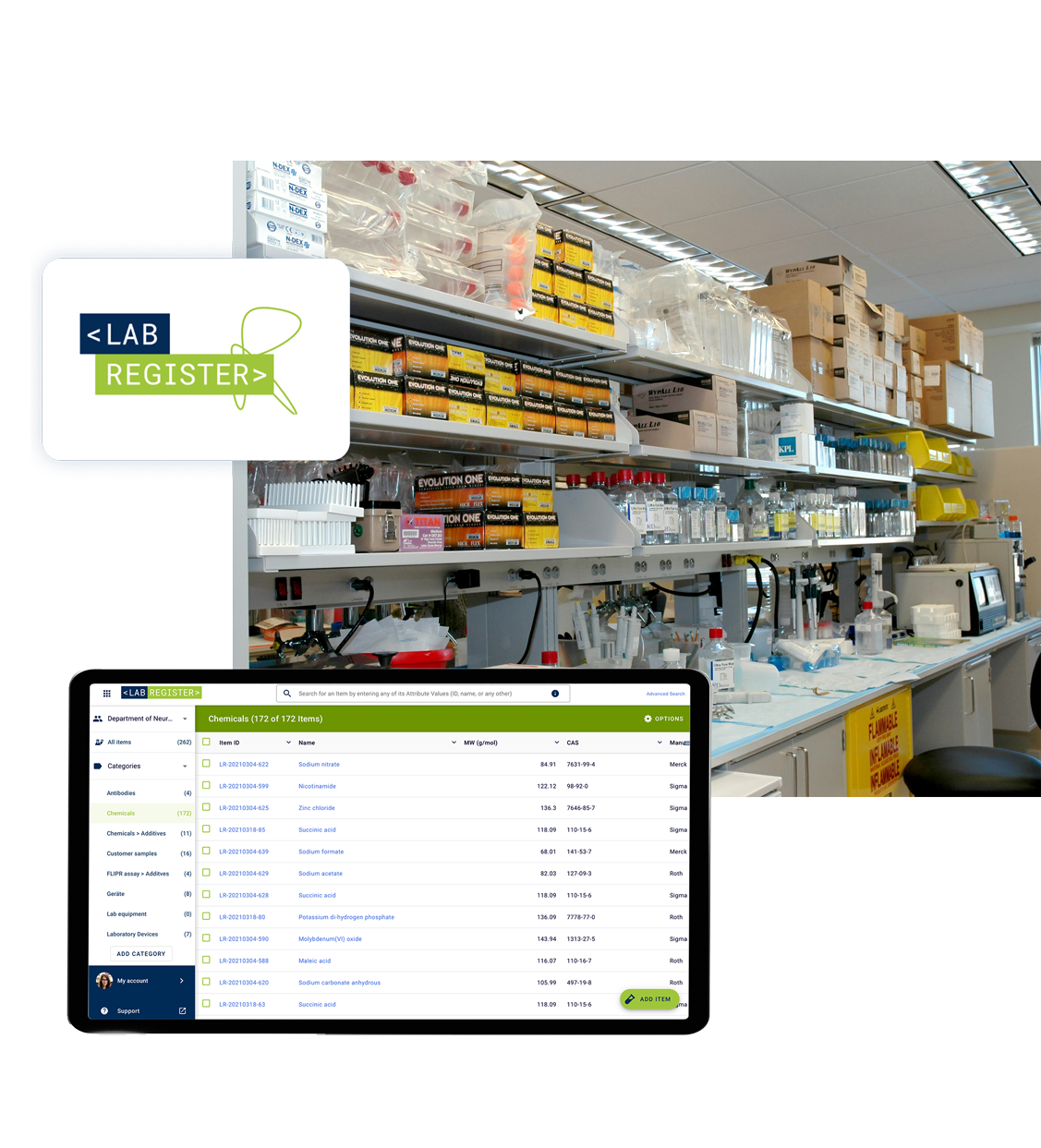
Take Control of Your Inventory
Say goodbye to clutter and confusion! Labregister simplifies inventory management, helping you manage and track samples, chemicals and equipment—all in one easy-to-use platform.
-
Centralized modern lab inventory
-
Seamless integration with Labfolder
-
Effortless & intuitive
Connect. Monitor. Automate.
-
Vendor-independent device connectivity
-
Enterprise-ready deployment
-
Tailor-made solutions

Mobile and Smart, Your Lab Companion.
-
Protocol guidance with data prompting
-
Picture taking & barcode scanning
-
Secure & compliant

AI at the core
Get the most advanced AI capabilities.
Handoff routine tasks and focus on high-value work. We are bringing AI to our product suite, trained on scientific content. Labforward’s expertise in AI combines accuracy with full compliance.
Network of partners
The connectivity puzzle cannot be solved alone.
From device manufacturers to global pharma companies, our strong network of partners helps deliver complete lab connectivity solutions. Together, we're solving tomorrow's digital challenges today.






















Our accomplishments:
Connected devices at top pharma.
Users in 20+ countries
Reclaimed each week
Industries and use cases
Lab-focused, versatile by design.
Labforward’ solutions bring smart digitalization to diverse laboratory environments, scaling from university labs to global enterprises. We work across various industries with unique demands, adapting to each environment. Our vendor-agnostic integration and open API architecture transform laboratory operations across the lab industry.
Customer Impact
We couldn’t say it better.
"Labfolder has completely replaced the physical lab journal we were using. It enables us to work more efficiently and streamlines a lot of our workflow."
Dr. Jochen Beck, Senior Scientist
Geistlich Pharma
"We work in a very collaborative setting, and Labfolder is the tool of choice when it comes to bringing pieces of a project together. Labfolder offers elements I need to structure and tag my data with, and the ability to easily share it amongst colleagues in a manner that I see fitting."
Dipl.-Ing. Ingo Przesdzing, ELN Program Manager
Charité
“With Laboperator we monitor and automate R&D processes in our chemical laboratories. Introducing vendor- and interface independent technology is a crucial foundation for the vastly adoption of laboratory software solutions.”
Sebastian Christ, Digital Lab Consultant
Bayer
“We realized that even if we have an ELN, scientists don’t stop to enter their data. Labtwin solves that for us with hands-free digital documentation.”
Marija, Scientist
Center of Analytical Innovation, dsm-firmenich
“Labtwin has made my documentation more accurate and my experiments more reproducible.”
Adam, Associate Principal Scientist
Pharmaceutical industry
“Labtwin has made my documentation more accurate and my experiments more reproducible.”
Adam, Associate Principal Scientist
Pharmaceutical industry
Learn more about the Lab of the Future
The Lab of the Future is happening. Expand your knowledge.

Case Study
Improving lab efficiency: how dsm-firmenich leveraged Labtwin's Digital Lab Assistant
dsm-firmenich achieved 100% real-time digitization of lab data and resulted in a 16% increase in data capture.

White paper
Digital lab management in ISO certified laboratories
Read how Labfolder streamlines ISO compliance for labs through digital management and documentation.
.jpg?width=369&height=209&name=Image%20(5).jpg)
Follow us on LinkedIn
Follow us on LinkedIn to stay up to date.
Explore Labforward.
-
Streamline your workflow with interoperable vendor-agnostic solutions
-
Make sure your data is safe and compliant
-
Accelerate your experiment cycle with AI-powered solution


