Stop hunting for signatures, speed up your review process today!
Take control of your review and approval processes with Review workflows plus — the smart electronic signature solution built into our Electronic Lab Notebook (ELN) Labfolder to simplify compliance and collaboration.
Create custom workflows to streamline your review processes, ensuring compliance with FDA Title 21 CFR Part 11 for your scientific documentation.
Designed for teams across R&D, QA and regulatory affairs, Review workflows plus helps your team move faster without compromising compliance or data integrity.
Request A Demo
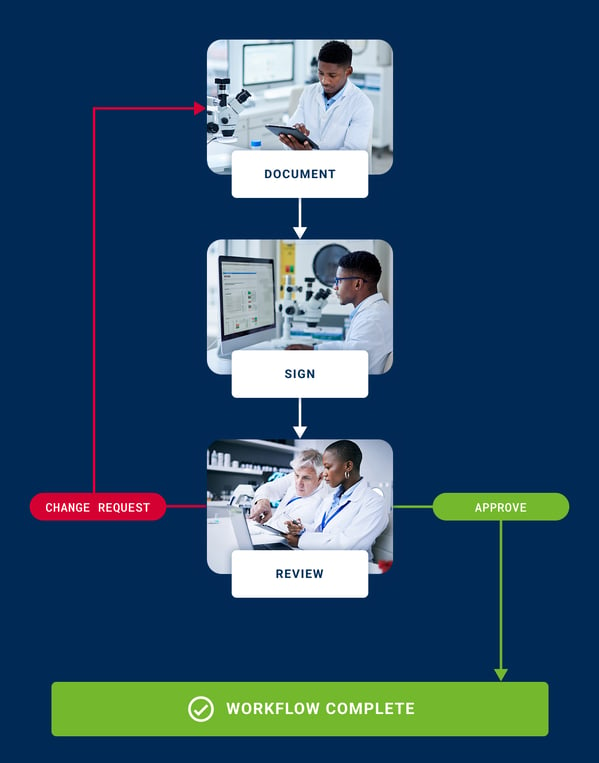
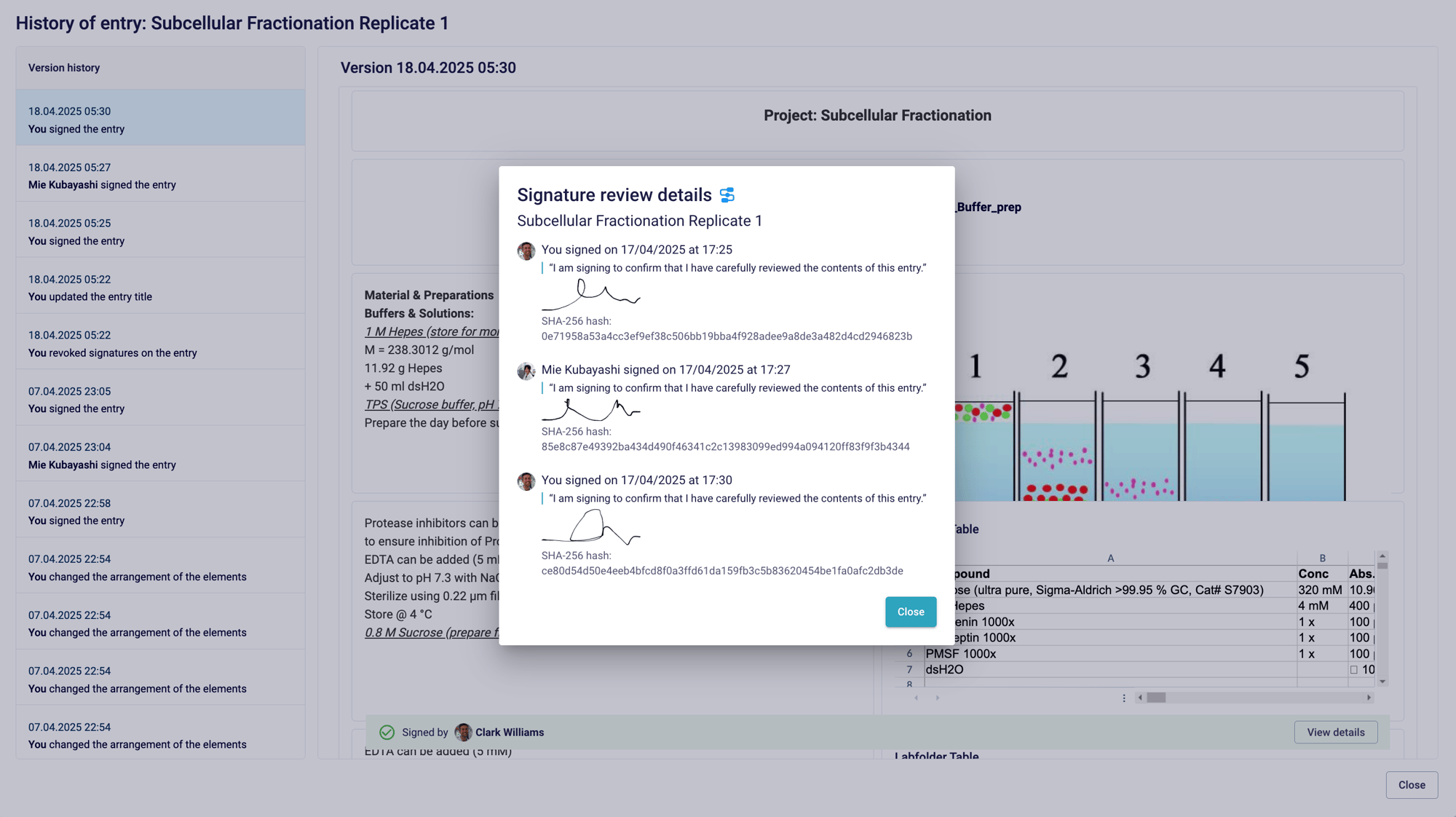
Review workflows plus enables tailor-made witnessing processes
-

Tailored workflows for effortless entry review
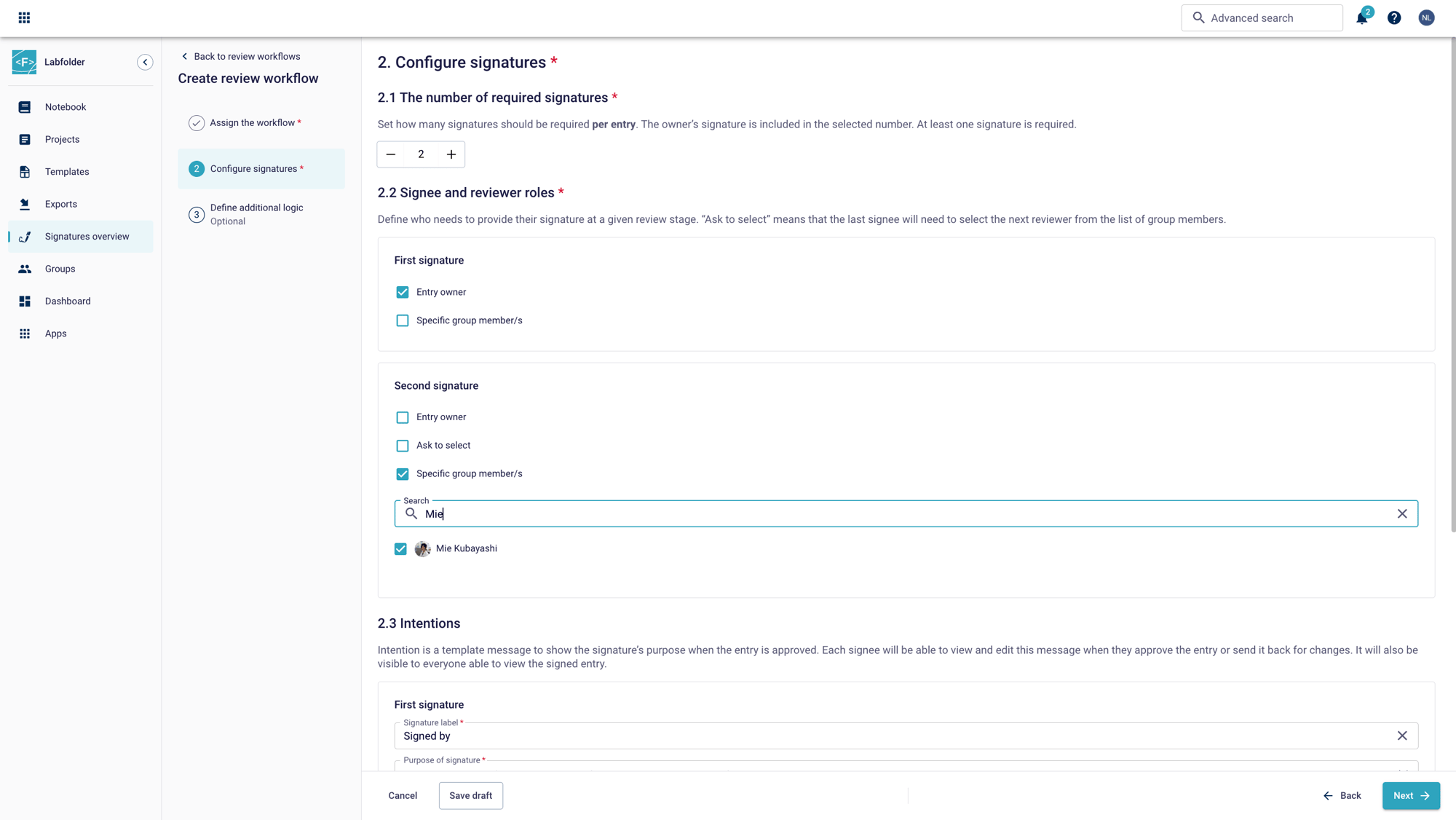
Set up project-specific workflows with Review workflows plus that support up to 10 witnesses. Select between the entry owner or specific group members or allow the signee to select the next reviewer to suit your unique process. Plus, enable your team to quickly state the purpose of each signature using predefined intentions.
-

Custom actions for individual workflows
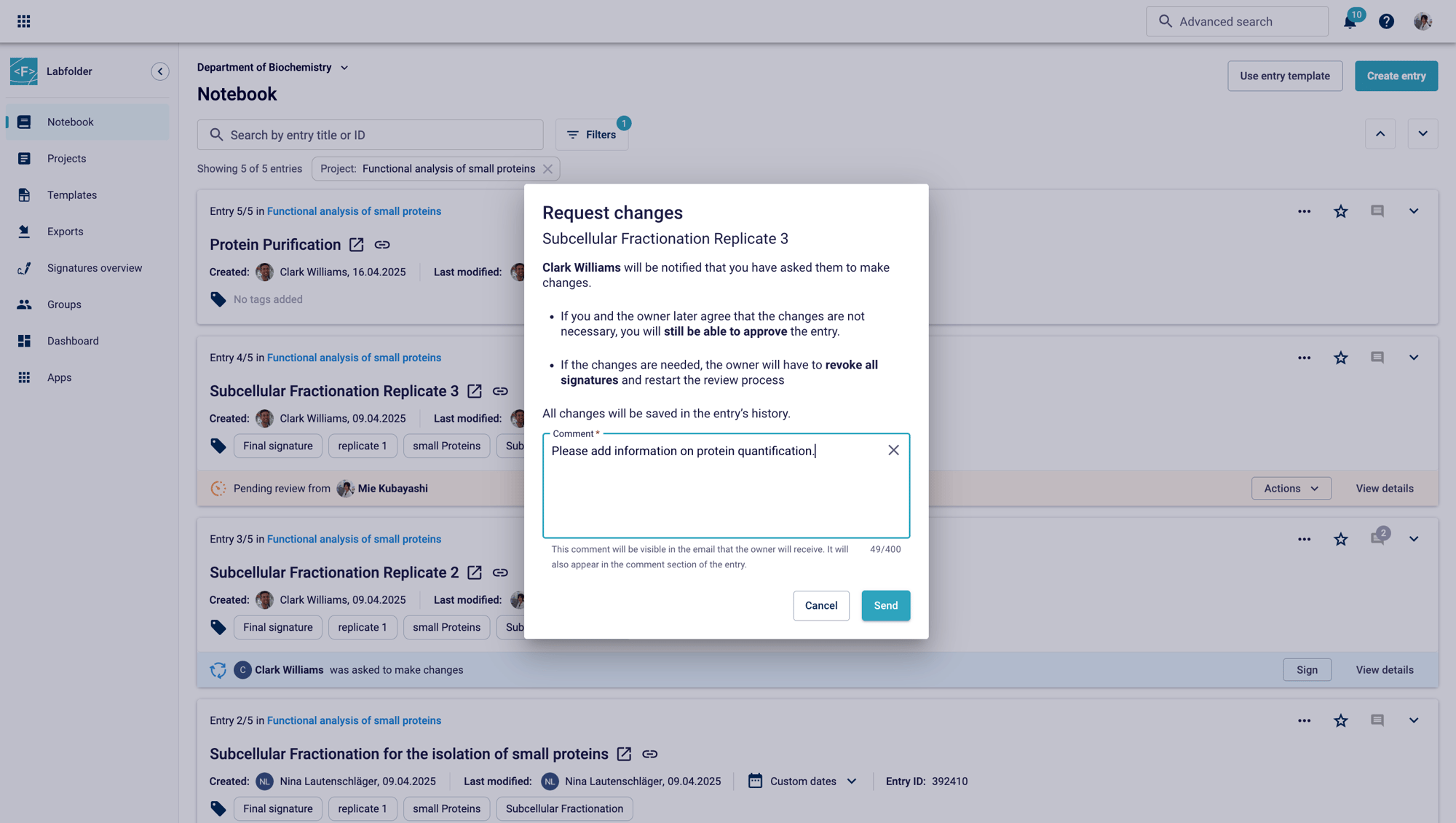
Predefine comment templates and tags during setup to streamline the review process. Set individual intentions to show each signature’s purpose within the review process. Once approved, entries can be seamlessly moved to another project, simplifying cross-departmental collaboration.
-

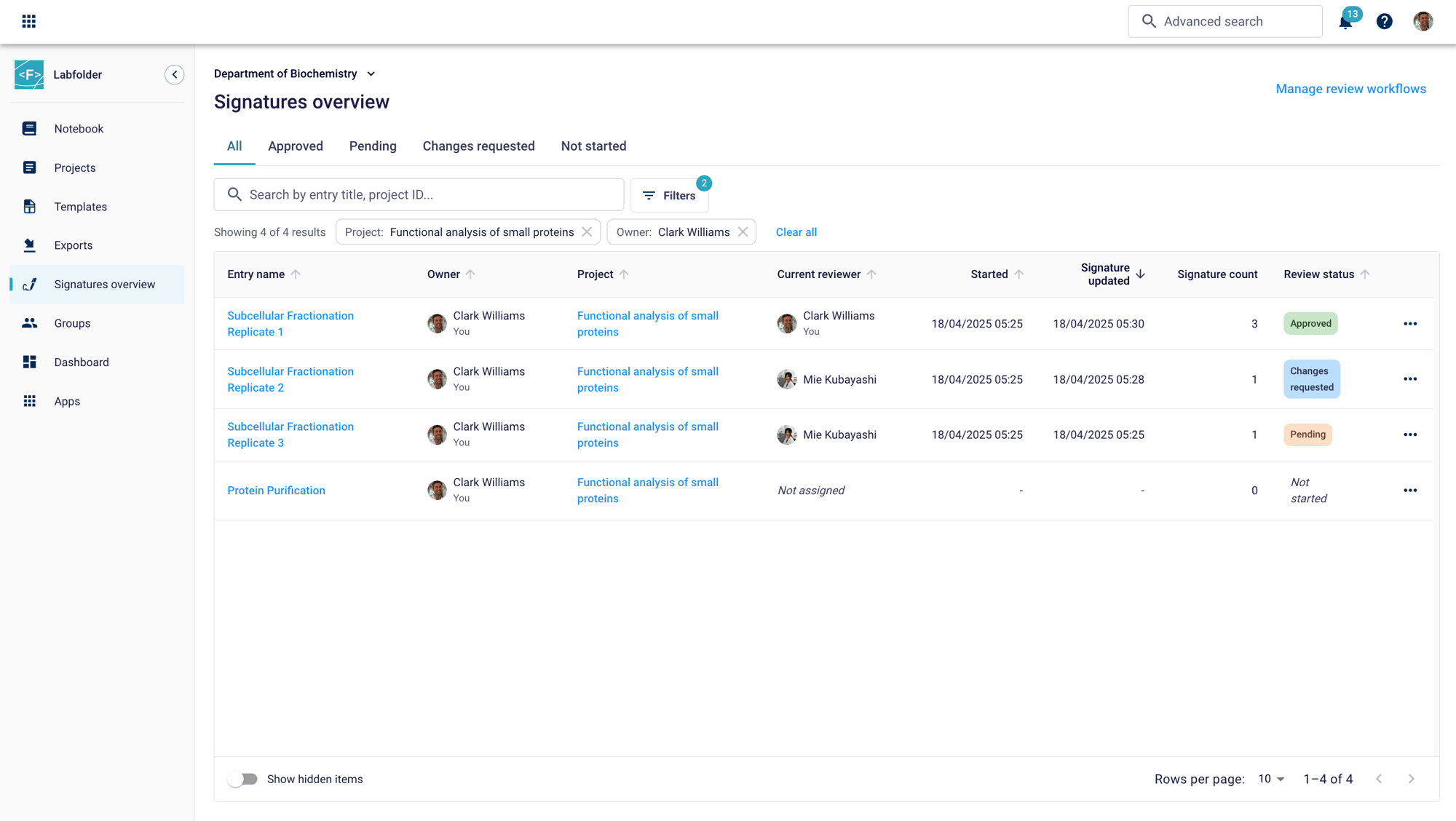
Real-time progress monitoring
Custom tags like “Approved” or “Change Request” make it easy to search and track reviewed entries. Follow ongoing processes by filtering for owner, projects or reviewers or use the selective viewing option to easily pinpoint approved entries, pending signatures or change requests.
-

Full compliance and data integrity
Labfolder’s electronic signatures comply with FDA regulations CFR 21 Part 11. Each entry is secured with a unique hash-sum and timestamp, ensuring data integrity and providing a clear audit trail for regulatory purposes.
Choose the right review process for you.
Review workflows
Standardized two-step procedure with signatory & witness
Not using Labfolder Advanced yet?
Most popular
Review workflows plus
For tailor-made witnessing processes
€13
€ / $ / £€4.25
€ / $ / £per user/month
DISCOVER YOUR BENEFITS
Compare our apps.
Find the signature app that meets your needs and fits your scientific research data management.
Review Workflows
Review Workflows Plus
Review Workflows
Review Workflows Plus
What you’ll get
Start with Labfolder.
-
Explore how Labfolder works
-
Talk to our expert to find the right solution for your team
-
Optimize your workflow with our electronic lab notebook
